
Click the link below the picture
.
In 2017, Ashley Moffett, a reproductive immunologist, walked to the pharmacy near her laboratory at the University of Cambridge, UK, to buy a pregnancy test. But it wasn’t for Moffett. Her postdoc, Margherita Turco, had created what she thought might be the first cluster of cells capable of mimicking the tissue of the placenta — a placental organoid. But she needed a way to be sure.
“We must do a pregnancy test on them,” Moffett said.
If Turco was correct, the miniature ball of cells she had created would secrete HCG, the hormone that triggers a positive pregnancy test. “I took the stick, put it in, and it was positive,” says Turco, now a reproductive biologist at the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland. “It was the best celebration.”
Scientists make organoids such as this by coaxing stem cells to grow in a jelly-like substance and to self-assemble into clumps of tissue. The typically hollow or solid balls of cells don’t look anything like real organs. But they do take on key aspects of the organ that they’re meant to represent — liver, brain, lung, or stomach, for instance.
The mini-organs have the advantage of being more realistic than a 2D cell culture — the conventional in vitro workhorses — because they behave more like tissue. The cells divide, differentiate, communicate, respond to their environment and, just like in a real organ, die. And, because they contain human cells, they can be more representative than many animal models. “Animals are good models in the generalities, but they start to fall down in the particulars,” says Linda Griffith, a biological engineer at the Massachusetts Institute of Technology in Cambridge.
Over the past decade, organoid research has exploded. Researchers have used them to study early brain development, test cancer therapies, and much more. And these 3D models stand to become even more crucial as US agencies, including the National Institutes of Health, the Food and Drug Administration, and the Environmental Protection Agency, aim to move away from animal testing.
Now, researchers are using organoids to study female reproduction, an area in which animal models can be especially limited. Lab mice, for example, don’t menstruate. And their placentas don’t develop in the same way as human placentas do. That challenge, along with a historical lack of funding for women’s health research, has left basic questions unanswered.
“I really see it as a powerful model to do science,” says Mirjana Kessler, a cell biologist at the Ludwig Maximilian University of Munich in Germany, who has developed an organoid that mimics the fallopian tube and a biobank of ovarian cancer organoids.
Organoids of the placenta, endometrium, ovary, and vagina could help to reveal how these organs function, and what happens when things go awry.
“There’s so much work to do to understand the normal biology,” Turco says.
The placenta invades
The placenta plays a key part in maternal health during pregnancy. Humans aren’t the only species that develops a placenta, but the “human placenta is quite different than most other species, even primates, actually, apart from apes”, says Moffett. Mice and humans, for example, both have placentas that invade the uterine lining, but the timing of development and the depth of invasion differ. Exactly what happens during the early days of placental development is still unclear, but problems at this stage can have serious consequences later.
One of the placenta’s first jobs is to create a link between the mother and the developing embryo. To do this, the placenta invades the spiral arteries that feed the uterus. The invasive cells open up the arteries, “essentially making a channel so that mom can provide what she needs through her blood supply”, says Victoria Roberts, a developmental biologist at the Oregon National Primate Research Center in Beaverton. (Nature recognizes that transgender men and non-binary people might have female reproductive organs and might become pregnant. ‘Mother’ is used in this article to reflect language used by the field.)
The process can be deadly if it goes wrong. If the placenta invades too deeply, a condition called placenta accreta, the expectant mother can lose too much blood during birth. And if the organ doesn’t invade deeply enough, then the fetus might not get enough nutrients to sustain its growth.
Shallow invasion can also impact the mother’s health. When the placenta doesn’t get enough blood, research suggests it can become inflamed and secrete harmful factors into the mother’s blood that trigger pre-eclampsia, a condition characterized by protein build-up in the blood and dangerously high blood pressure. Worldwide, 2–8% of pregnant people develop the condition. “It’s a very serious pregnancy complication that goes silent and undetected until very late into pregnancy,” says Quinton Smith, a chemical engineer at the University of California, Irvine. The only way to cure the condition is to deliver the baby, even if that means a preterm birth.
To better understand the condition, Smith, Turco, and other researchers are using organoids made of placental cells called trophoblasts to model the molecular processes involved. Turco is focused on the basic biology of how invasion is regulated, a process that seems to be controlled by both the fetus and the mother. “It’s got to be a compromise,” Moffett says. “It’s an absolute dialogue.”
That dialogue seems to be happening between the placenta and the uterine lining. As a case in point, when an embryo implants somewhere the lining doesn’t exist — on a scar left by a previous caesarean delivery or in a fallopian tube, for example — “there’s no control of the invasion at all”, Turco says.
Research suggests that immune cells called uterine natural killer cells have a key role in this conversation. The cells don’t kill but instead send out chemical signals that help to regulate the invasion of the uterine lining.
.
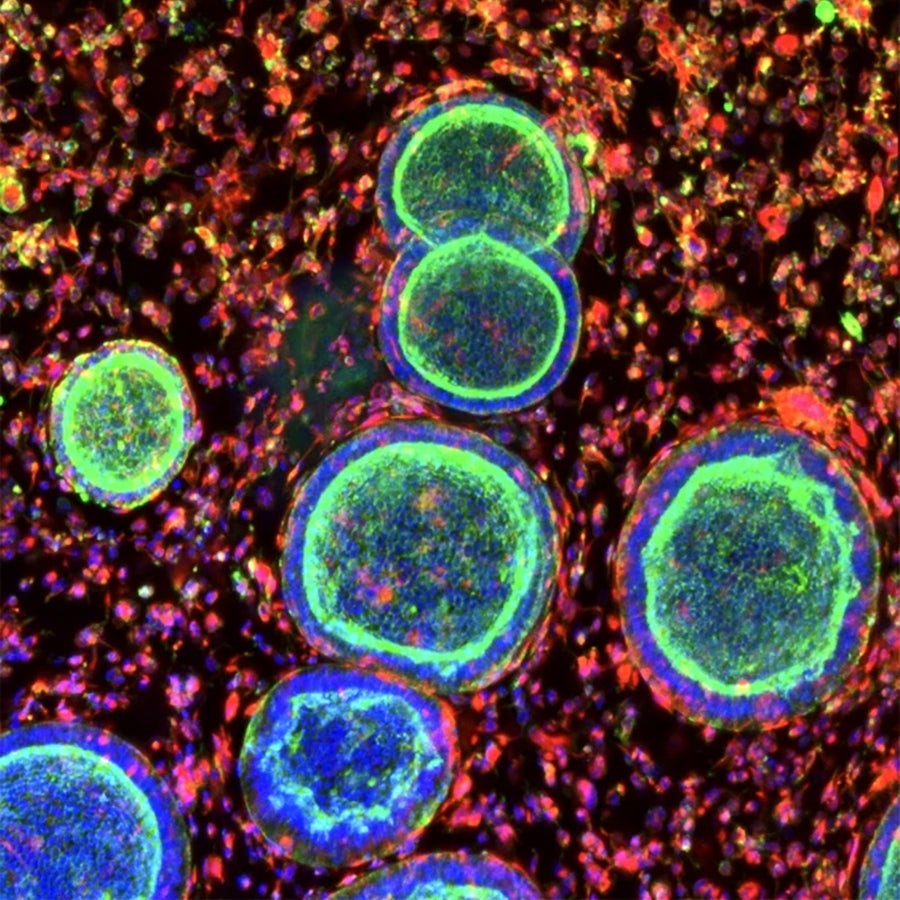
Cell clusters that resemble the uterine lining grow with support cells in a synthetic hydrogel. Juan Gnecco, Linda G. Griffith
.
.
Click the link below for the complete article:
.
__________________________________________
Leave a comment